Minimally Invasive Technology Temporarily Reverses Blood Flow in the Brain to Prevent Devastating Strokes
Surgical Care Associates working at Baptist Health Louisville is the first in state to offer an innovative new treatment for patients at risk for stroke due to blockages in the neck arteries known as carotid artery disease. The minimally invasive procedure, called TransCarotid Artery Revascularization or TCAR, utilizes a new FDA-approved neuroprotection system that temporarily reverses blood flow in the artery during the procedure. Dangerous bits of plaque and blood clots that could dislodge and otherwise travel to the brain and cause a stroke are safely diverted away while a dedicated transcarotid stent is inserted to open and stabilize the blockage. 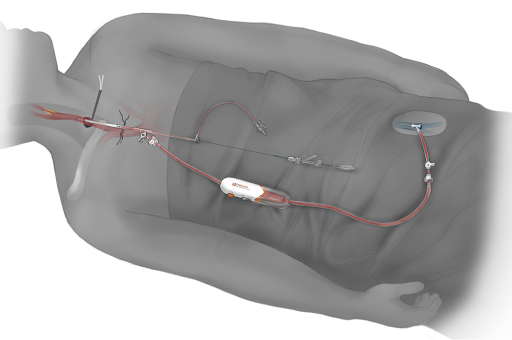
Prior to TCAR, the main treatment option for severe carotid artery disease was an open surgical procedure called carotid endarterectomy. The surgical technique allows for protection of the brain during the procedure, but the large incision leaves a visible scar the length of the neck and carries risks of surgical complications including bleeding, infection, heart attack, and cranial nerve injuries that can cause issues with swallowing, speaking, and sensation in the face.
Bradley Thomas, a vascular surgeon at Surgical Care, is amongst the first in the country to gain expertise with the TCAR procedure. “TCAR gives me the excellent neuroprotection I expect from carotid endarterectomy, but the procedure is less invasive which has real benefit to the patient. They recover quickly with less pain, and the risks of both minor and major complications are significantly decreased,” explained Dr. Thomas. “It represents a modernization of carotid repair.”
Results from clinical trials of TCAR were so compelling that the Society for Vascular Surgery (SVS), in collaboration with the Food and Drug Administration (FDA) and the Centers for Medicare and Medicaid Services (CMS), recently launched a novel program called the TCAR Surveillance Project. This program provides expanded insurance coverage of TCAR procedures for Medicare beneficiaries while allowing individual hospitals and the society to track quality benchmarks.
While minimally invasive stenting procedures have become commonplace to treat other vascular diseases in the heart, abdomen and legs, they have not historically been used broadly in the carotid arteries. This is due to the inherent risk of stroke as stents and other endovascular devices are navigated from a typical entry point in the patient’s femoral artery in the groin (transfemoral) all the way through the body up to and across the blockage in the neck. Along the way, friable vascular disease can get dislodged and travel to the brain and cause a stroke. Clinical trials of transfemoral carotid stenting showed a twofold risk of stroke relative to carotid endarterectomy. “TCAR starts at the neck, not the groin, which bypasses a big source of stroke risk historically associated with transfemoral carotid stenting. After gaining transcarotid access, we then initiate temporary blood flow reversal as the first step of the TCAR procedure to make sure the brain is adequately protected before placing the stent. It’s a very logical yet ingenious technology,” explained Dr. Thomas.
Every year, 15 million people worldwide suffer a stroke, also known as a “brain attack.” Nearly six million die and another five million are left permanently disabled. Stroke is the second leading cause of disability globally. Carotid artery disease is estimated to be the source of stroke in up to a third of cases and there are 400,000 new diagnoses of carotid artery disease made every year in the United States alone.[1],[2]
“TCAR is an important new option in the fight against stroke, and is particularly suited for the large portion of patients we see who are at higher risk of complications from carotid endarterectomy due to age, other medical conditions, or anatomic issues. Because of its low stroke risk, we think TCAR has the potential to become the standard of care for many patients,” said Dr. Thomas.
Baptist Health Louisville is one of less than 50 sites in the United States currently enrolling patients into the ROADSTER 2 post-approval study. ROADSTER 2 is a prospective, multi-center study designed to assess the real world treatment of patients at risk for stroke due to carotid artery disease with TCAR. The prospective study will include a minimum of 600 patients.
About TCAR with the ENROUTE Transcarotid Neuroprotection and Stent System
The ENROUTE® Transcarotid Neuroprotection (NPS) and Stent System from Silk Road Medical, Inc. are the first devices designed and FDA-approved specifically for TCAR. The ENROUTE Transcarotid Stent is indicated for use in High Surgical Risk patients and is intended to be used in conjunction with the ENROUTE Transcarotid Neuroprotection System (NPS). The ENROUTE Transcarotid NPS is used to directly access the common carotid artery and initiate high rate temporary blood flow reversal to protect the brain from stroke while delivering and implanting the ENROUTE Transcarotid Stent.
[1] http://www.world-heart-federation.org/cardiovascular-health/stroke/
[2] https://vascular.org/patient-resources/vascular-conditions/carotid-artery-disease